In the reaction MgCl2MgCl2 the correct half-reaction for the oxidation that occurs is A. 2Lis Cl2g - 2LiCls As the reaction takes place the Cl2g will.

1 Which Statement Best Describes An Oxidation Reduction Reaction 1 Point A A Chemical Reaction In Brainly Com
ACu2 BZn CZn2 DCu.
. B The reaction occurs in a voltaic cell and absorbs energy. Third option is the correct one. 1Which statement best describes an oxidation-reduction reaction.
Step 6 NADH is produced NAD is reduced to NADH accepting H and high energy electrons 2 Step 10 ATP is produced via oxidation of phosphoenolpyruvate into pyruvate 3. Redox it is a chemical reaction in which there is the occurrence of oxidation and reduction of atoms of substances chemical species present in the process. A chemical reaction in which there are fewer products than reactants.
Chemistry 19092021 2310 NetherisIsTheQueen. Both charge and mass. 1Which statement best describes an oxidation-reduction reaction.
Memorize flashcards and build a practice test to quiz yourself before your exam. Since cation is positively charged and the oxidation of an atom is zero thus the oxidation no. A chemical reaction that involves oxygen C.
Base your answer to the following question on the diagram below which represents a chemical cell at 298 K and 1 atmosphere. Start studying the Oxidation-Reduction Quiz flashcards containing study terms like Which describes the oxidizing agent in a chemical reaction Given the reaction below which is the oxidized substance. A chemical reaction that involves oxygen.
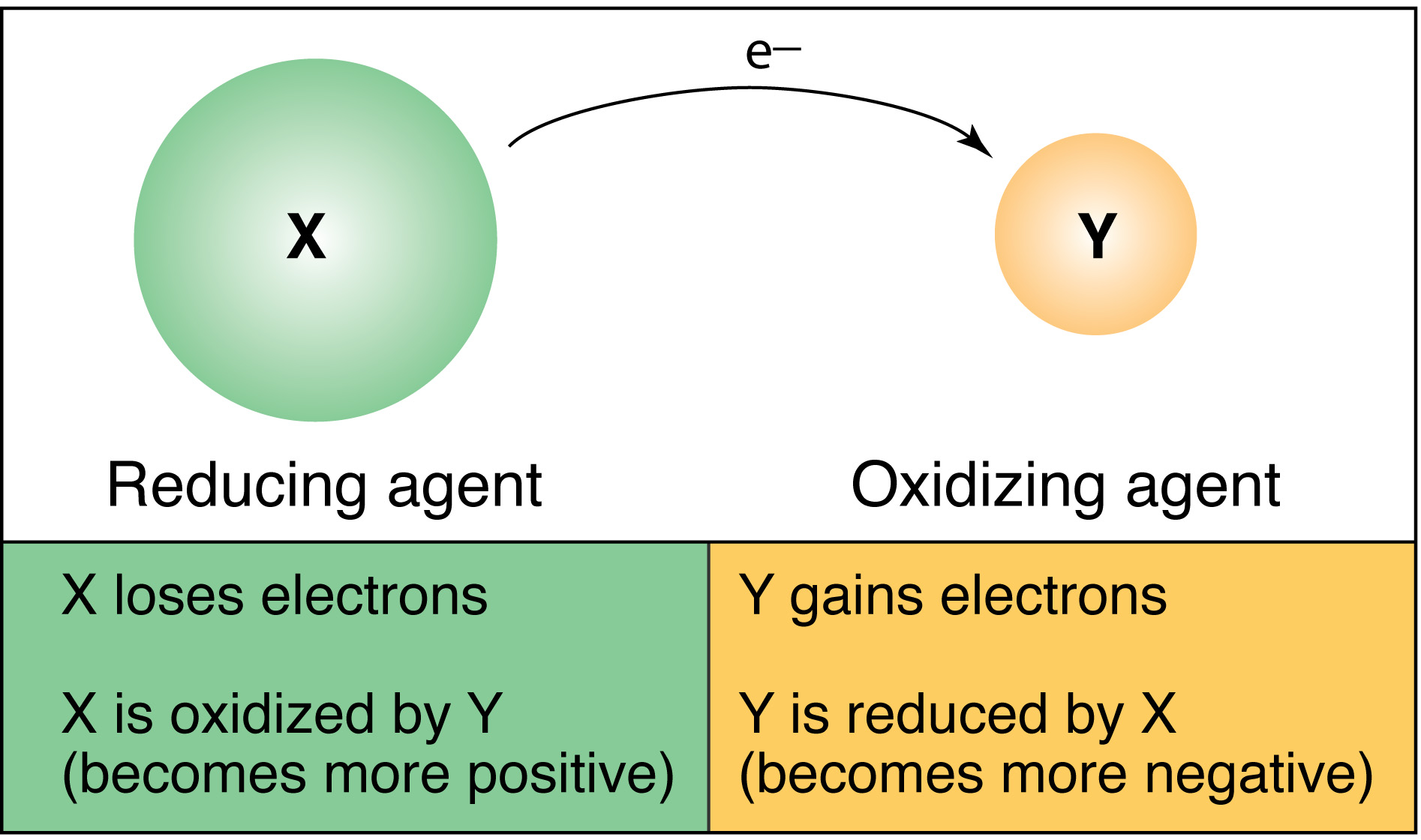
Silver ion Ag is a stronger oxidizing agent than copper ion Cu2 and copper metal is a stronger reducing agent than silver. A chemical reaction in which electrons are released from the system. These reactions involving electron transfers are known as oxidation-reduction or redox reactions.
Which of the following statements best describes an oxidation-reduction reaction. A The reaction occurs in a voltaic cell and releases energy. A chemical reaction in which electrons are released from the system D.
The sharing of a pair of electrons between two atoms a relatively strong bondb. If there is formation of a precipitate the reaction is an oxidation-reduction reaction. All of the answers are correct.
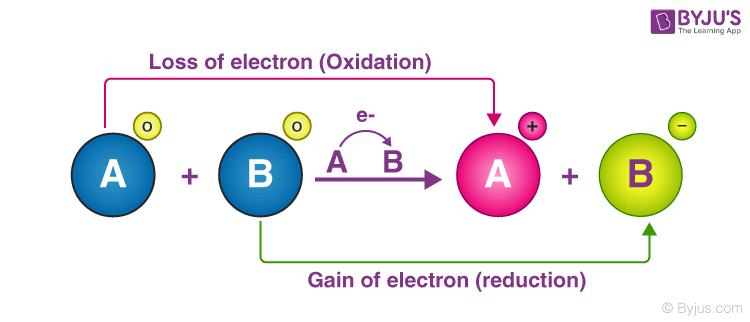
Oxidation is the loss of electrons by an atom of a chemical species while reduction is the gain of electrons by an atom of a chemical species. O If there is formation of a precipitate the reaction is an oxidation-reduction reaction. Which quantities are conserved in all oxidation-reduction reactions.
Which of the following statements best describes an ionic bond. A chemical reaction in which electrons are transferred between reactants 2Beryllium Be has four electrons. Which statement best describes the oxidizing and reducing abilities of the reactants.
Is reduced ie electrons are accepted and thus it involves reduction. CIt uses radioactive nuclides. O O O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction If there is formation of salt and water the reaction is an oxidation-reduction reaction.
But when an element is reduced it. Posted 25 days ago. Cl2 2e 2Cl C.
Thus during an oxirreduction reaction electrons move from the species. A chemical reaction in which there are fewer products than reactants B. Hence we conclude that the correct answer to this question is option B.
The sharing of a pair of electrons between two atoms a relatively weak bond. Mg Mg2 2e D. 4Al 302 2Al2O3 In this reaction aluminum is oxidized and oxygen is reduced In this reaction aluminum is reduced and oxygen is oxidized In this reaction th aluminum and oxygen are oxidized This is not an.
O If there is formation of salt and water the reaction is an oxidation-reduction reaction. Which statement best describes how a salt bridge maintains electrical neutrality in the half cells of an electrochemical cell. BIt undergoes a spontaneous redox reaction.
When an element is oxidized it loses electrons. Conversion of cation to atom involves gaining of electrons. The attraction between two charged atoms a relatively weak bond in an aqueoussolution.
O If there is no change in the oxidation states of atoms from reactants to products the reaction is an oxidation- reduction reaction. A chemical reaction that involves oxygen. The reaction that takes place in a chemical cell is best classi ed as A.
Which statement best describes the reaction represented by the equation2NaCl 2H2O electricity -- Cl2 H2 2NaOH. This is the basis of redox reactions. 1Which statement best describes an oxidation-reduction reaction.
C The reaction occurs in an electrolytic cell and releases energy. In this reaction iron is reduced and copper is oxidized In this reaction both iron and copper are oxidized Which statement best describes the following reaction. Question 7 1 Poi All sugars fermented by bacteria and yeast.
Step energy transferring from 1 3-bisphosphoglycerate to ADP produces ATP 4. But when an element is reduced it gains electrons. The sharing of a.
DIt requires an external energy source. Because of this in many cases H 2 O or a fragment of an H 2 O molecule H or OH in particular can participate in the redox reactionAs such we need to learn how to incorporate the solvent into a balanced redox equation. Which equation represents the half-reaction that takes place at.
However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. O If there is a change in the oxidation states of atorns from reactants to products the reaction is an oxidation- reduction reaction O If there is formation of salt. 11Which statement describes one characteristic of an operating electrolytic cell.
Mg Cl2 Mg2 2Cl Which best identifies why the rusting of an iron nail in the.
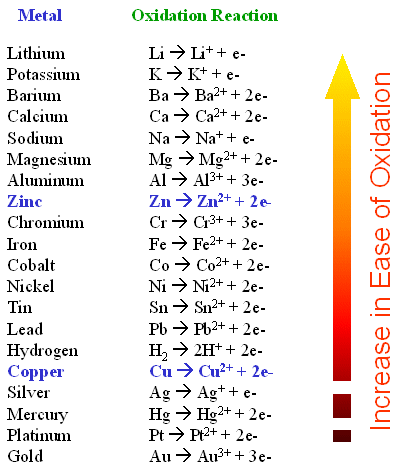
Other Oxidation Reduction Reactions

Chemistry Reduction And Oxidation Reactions Wikiversity
0 Comments